Don’t inherit supplier problems. Monitor their compliance at all times, and learn of issues before they become yours.
Introducing Supplier Intelligence – Ryden’s AI platform allows you to track the compliance of all your suppliers in real time. You can even push your own Policies and Procedures and Record Types to your suppliers, and validate they are following those.
Use Cases

Proactive Issue Tracking
Traditional compliance finds problems after they’ve already caused damage. Ryden’s system flags issues at the earliest sign of non-compliance, preventing recalls, penalties, and lost revenue. Make sure your entire supply chain is in compliance, especially when a new or updated regulation occurs.

Push Your Processes to Suppliers
Extend your own internal procedures to your suppliers with a click. Instead of waiting for alignment, you can enforce your exact quality standards across the supply chain. This dramatically reduces discrepancies that typically surface during FDA or ISO audits.

Comprehensive Supplier Dashboard
A centralized dashboard transforms compliance from a reactive, document-heavy process into a proactive, data-driven operation. Executives gain immediate insights into global supply networks without waiting weeks for manual reports.

On-Demand Assessments
Request new compliance checks in real-time, eliminating the long cycles of scheduling audits. This agility means suppliers can be re-validated after changes, saving both sides significant costs in rework and downtime. Assessments can be custom, manufacturer specific, or even supplier specific.
Key Features & Impact
Compliance Scores for All Suppliers
Instantly see the compliance health of every supplier. This eliminates the guesswork of traditional audits where only a handful of suppliers get audited. Continuous visibility reduces the need for expensive site visits and audits.
Facility and Requirement-Level Scoring
Drill into specific facilities or even down to individual regulatory requirements. This precision targeting saves countless hours during investigations and ensures you can pinpoint weaknesses before they escalate into violations.
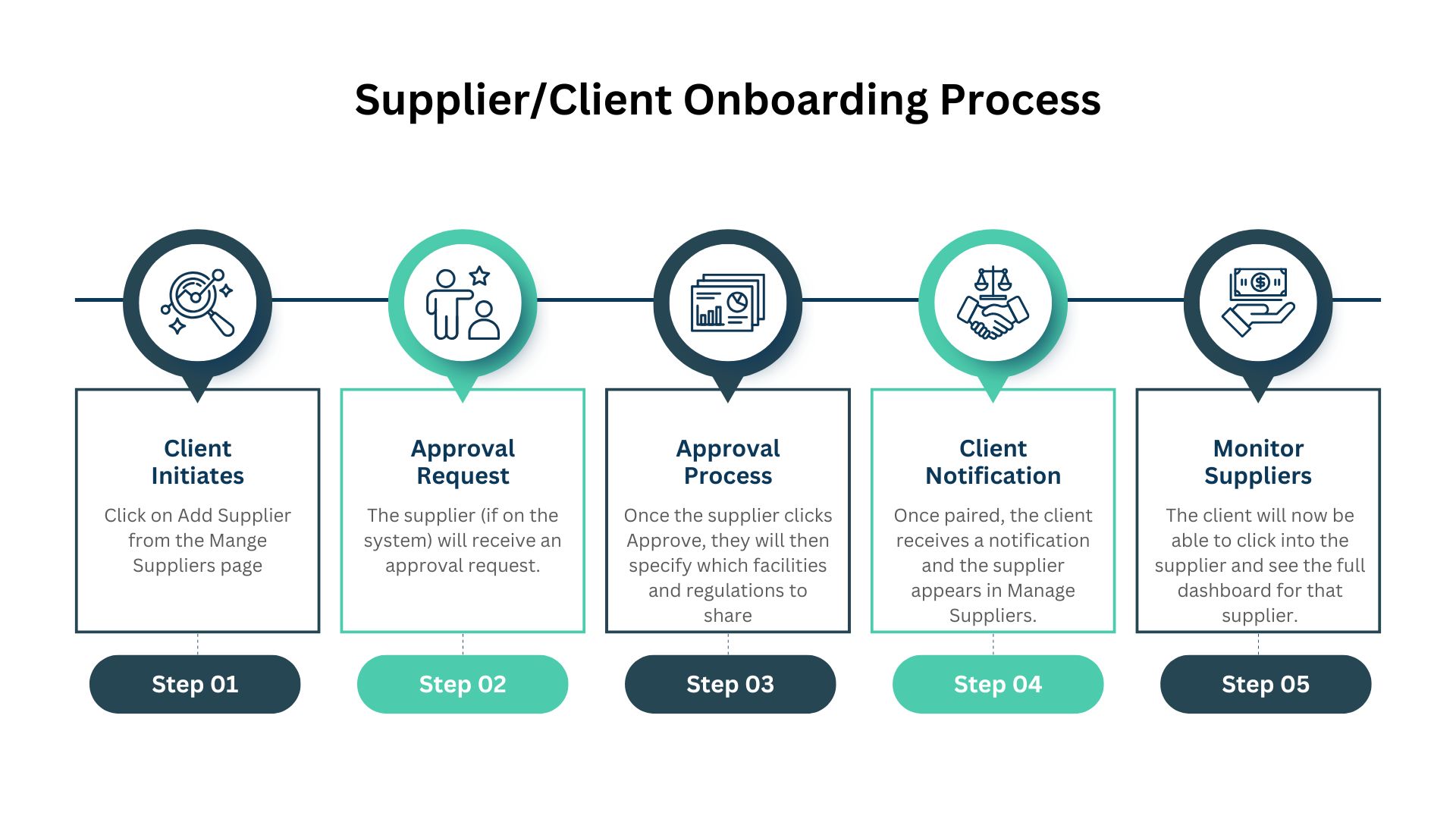
Supplier Request System
Request that a supplier becomes visible with a simple workflow. This removes the endless back-and-forth emails and contracts that slow down compliance onboarding. The approval process saves weeks of negotiation time per supplier.
Data Segregation
Suppliers never see your internal data, and you never see theirs. This strict separation protects confidentiality while still allowing transparency in scoring. It removes costly legal barriers to data sharing and accelerates collaboration.
Role Segregation
Assign specific facilities, requirements, and suppliers to individual users. This reduces the risk of error, protects sensitive data, and allows large organizations to scale compliance oversight without creating bottlenecks.
Value for companies of all sizes and number of suppliers

Startups
If your company is new to the Life Science space or just getting ready to take a product to market; make sure your suppliers are not the cause of your submission failure.

Mid-size
Between reduced audit costs, less travel, reduced risk management, and aligning your suppliers to your standards, the cost and time savings will allow you to focus on what is important.

Enterprise
The more suppliers you have, the larger the savings. If you have a team that travels all over the world to validate compliance at your suppliers, this will greatly reduce the need for travel, as well as make you aware in real time of issues before they become a problem.
Be Proactive, not reactive
Don’t wait till the FDA is at your doorstep to do an audit. Let us schedule a demo of our solution and show you how we will change the way you handle compliance.
