Introducing Records Intelligence, an AI-driven engine that automatically evaluates real-world records (Incoming Inspection, DHR’s, complaints, CAPAs, DCOs/ECOs, inspections, training, validations, calibrations, and more) against your design requirements, QMS requirements such as procedures and Good Documentation Practices (GDP) – continuously, verifiably, at scale, and all in one place.
Use Cases

Faster ROI-measured in months
Automated trending, Management Review metrics, and slide outputs are produced for you-every cycle. Whether it is Mergers and Acquisitions, Pre-Submission, supplier audits, or Corporate audits, the saves will add up.

Escalations before defects matter
Receiving inspection paperwork can be reviewed before parts hit the dock; low-risk pathways like skip-lot or dock-to-stock become data-driven.

Audit-ready evidence-anytime
Individual record reports and cross-record trending are generated out-of-the-box and easy to verify and archive – no more messy spreadsheets.

Good Document Practices (GDP)
Verifies that all of your documents follow Good Documentation Practices – legible, attributable, contemporaneous, etc.
Key Features & Impact
Scope & ingestion
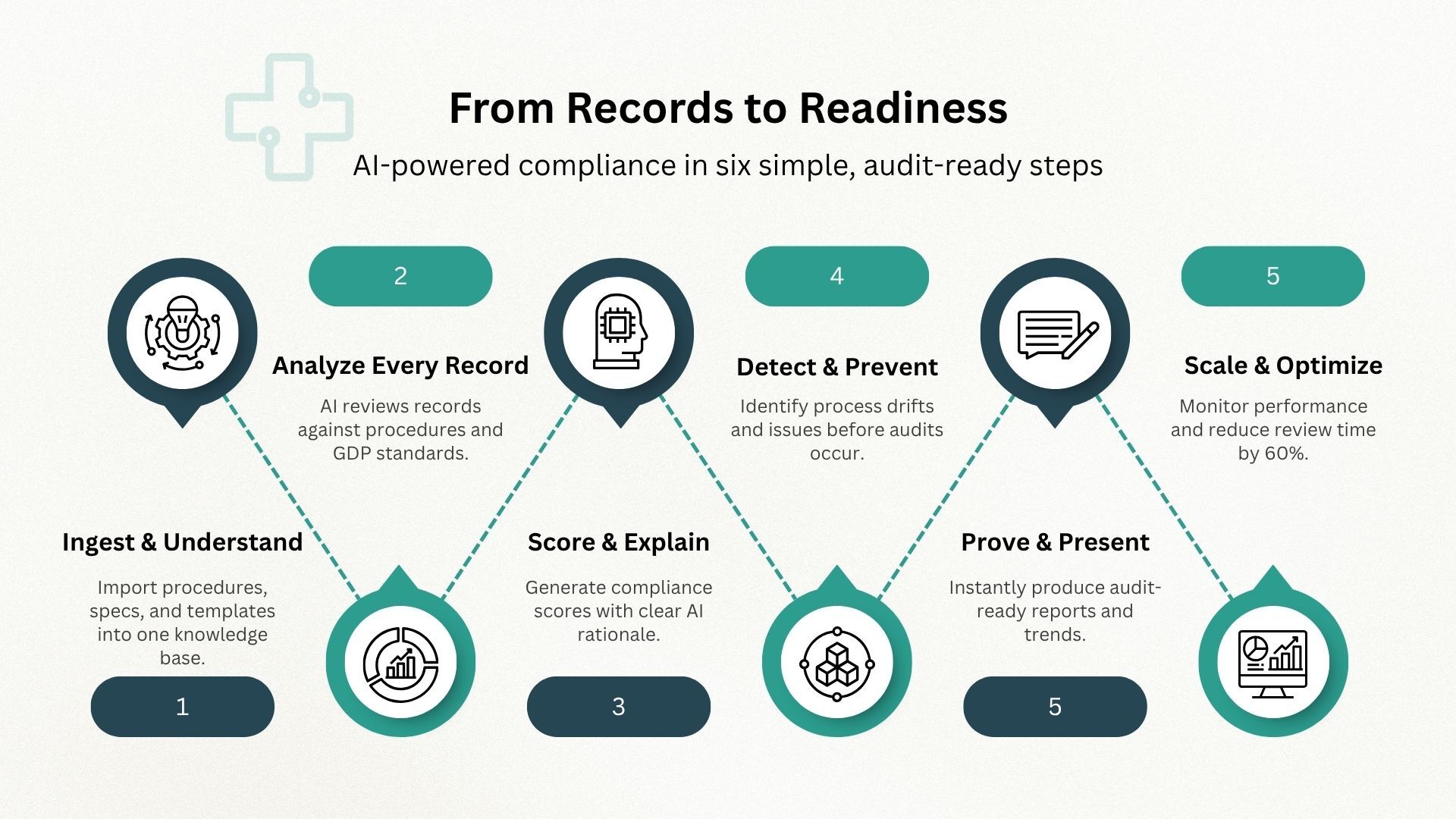
- Assesses real-world records against governing procedures, specifications/design requirements, and GDP expectations to save time and gain actionable insights.
- Accepts multiple record formats: eQMS electronic entries (with audit trails/signatures), electronic documents (PDF/Word/Spreadsheets, with/without fixed templates), and scanned handwritten forms (with performance caveats).
- Optional pre-approval/draft check so suppliers can validate and fix issues before sending.
- Ties records to the governing procedures and their mapped regulatory requirements (enables filtering “by law”).
Assessment modes & scale
- Three modes selectable at Facility- Record Type level: single record, random sampling (user-set sampling plan), or 100% verification (scales from 0 to 25,000+ records/month per facility).
- Works for both facility-level and product-level record types (product-specific scoping planned).
- Pre-upload or upload-at-assessment-time workflows supported.
Admin setup & governance
- Manage Records (Admin): define Facility–Record Type assessment, upload governing docs (policies/procedures/WIs/form templates), view associated laws, optionally upload an example completed record, and submit a setup request (ticketed release).
- Internal “Manage Record Assessments” tab to release assessment criteria per Facility–Record Type; visibility restricted to the specific facility.
- Question set is visible to users for transparency; edits/add/removals are handled via support tickets (no direct user editing).
What gets evaluated (four dimensions)
- Accuracy of outputs vs. specifications/requirements (e.g., did parts pass?).
- Completeness & inspection-readiness (required fields, approvals, data, attachments, common pitfalls).
- QMS process adherence (the right work performed as your procedures/specs require).
- GDP conformance (legible, attributable, contemporaneous, etc.).
Supported record types (starter set; user-extensible)
- Receiving Incoming Inspection, DHR, Complaints, CAPA, DCO/DCR, ECO/ECR, Operations Process Validation, Management Review, Nonconformities, DHF, RMF, Medical Device File, CSV, Training, QC Inspections, Calibration & Preventive Maintenance, plus user-entered types.
- User Defined Record Types are able to be uploaded into the system, to be used as a valid record type throughout the system, and against suppliers.
Outputs: Individual record report
- Structured, versioned PDF/UI report including: background/how-to-use, record name & revision, upload date, person, company/facility, question-set revision, documents assessed with names/revisions/dates, laws in scope, overall score + section scores (Accuracy, Completeness, GDP), per-question Y/N, AI rationale, and free-text Notes.
- “View record” and “download document” actions available from record-level details.
Cross-record trending & collections
- Collection report with totals, last-30-days counts, average scores (all-time and last 30 days), % fully compliant (overall and last 30 days), and average high/low-priority opportunity counts per record. Includes a time-series graph of overall score.
- Drill-downs: hyperlink directly to filtered tables of records behind any non-compliance metric.
- Trend status per record type: Increasing / Stable / Decreasing (10% default threshold over last 30 days vs. prior 3 months; facility-record tunable).
Value for companies of all sizes and number of suppliers

Startups
If your company is new to the Life Science space or just getting ready to take a product to market; make sure your documents follow your procedures, before submission.

Mid-size
Between reduced audit costs, increased ROI, and a confidence that you can bank on; Records Intelligence will check 100% of your documents, so you don’t have to.

Enterprise
Review time drops ~ 60%; paperwork issues are caught pre-arrival; parts can flow to inventory without queueing inspection when appropriate. Automate Receiving and Incoming Inspection, to reduce time and save money.
Be Proactive, not reactive
Don’t wait till the FDA is at your doorstep to do an audit. Let us schedule a demo of our solution and show you how we will change the way you handle compliance.
